Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematologic malignancy with a high rate of recurrence following standard chemotherapy that continues to have a poor prognosis. While there have been numerous recent advances in the treatment of AML, seven days of continuous infusion cytarabine combined with three doses of an anthracycline medication (7+3) remains the most common backbone treatment for younger (<60) patients. Following 7+3 treatment, patients are typically assessed by a bone marrow biopsy 14-21 days after initial treatment, and 20%-30% of patients will have persistent disease at this time. This residual disease is typically treated with an additional round of the same induction treatment at a full or slightly reduced dose. Unfortunately, a complete response (CR) following re-induction is infrequent in intermediate and high-risk AML patients (CR rates of 35% and 18%, respectively). We have performed mass cytometry studies of AML cell cycle state and find that in patients with residual disease at day 14-21 of induction, leukemia stem and progenitor cells have a low proliferative fraction. We hypothesize that this low rate of proliferation reduces the effectiveness of re-induction therapy and could be countered using CPX-351, which is FDA-approved for treatment of therapy-related AML and AML with myelodysplasia-related changes. CPX-351 is a formulation of cytarabine and daunorubicin in a liposomal structure at an optimal 5:1 molar ratio that has been shown to be preferentially absorbed by hematopoietic cells and to persist in serum and bone marrow for significantly longer than traditional 7+3 chemotherapy. We hypothesize that the unique properties of CPX-351 will better target quiescent leukemia stem and progenitor cells and we thus designed a phase 1b/2 study to determine the safety and efficacy of CPX-351 as re-induction treatment for patients with intermediate and high-risk AML with residual disease after a first round of induction.
Patients and Methods
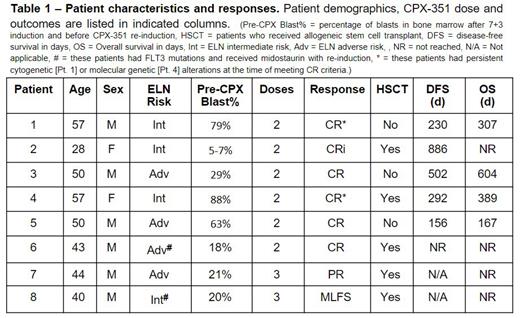
Eight patients with intermediate or high-risk AML who had failed to achieve hypocellular marrow 14-21 days following initial treatment with traditional 7+3 cytarabine and daunorubicin were treated with CPX-351 re-induction in the phase 1b portion of the study with a CPX-351 dose of daunorubicin 44 mg/m 2 and cytarabine 100 mg/m 2 given two or three times every 48 hours. Concomitant midostaurin (days 8-21) was allowed for patients with FLT3 mutations (Patients #1 and #8). Four patients were ELN intermediate-risk and four adverse-risk; mean blast count before re-induction was 45%. Patients were assessed weekly for signs of toxicity and for treatment response. Samples were collected from bone marrow biopsies performed before and after treatment for correlative mass cytometry studies using a 50-antibody panel to determine cell cycle state, intracellular signaling markers, and DNA damage response.
Results
Of the eight patients treated to date, six received two doses of CPX-351 and two received three doses. Of the six patients receiving two doses, five achieved a morphologic CR (two with residual genetic alterations), and one patient achieved a CRi. Two patients received three doses of CPX-351, with one achieving a PR and the other MLFS. Five of the eight patients were able to proceed to an allogeneic stem cell transplant. Consistent with previous studies of CPX-351, treatment was well-tolerated, almost all grade 3 or 4 toxicities were due to cytopenias (including two DLTs for delayed count recovery) and the only significant drug-related non-hematologic toxicity was a decrease in cardiac ejection fraction in patient #1. All treated patients survived >150 days and all were able to receive additional AML-directed therapy or HSCT after CPX-351 treatment. Due to slow accrual, hematologic toxicity, and the 100% CR/CRi rate in the two-dose group, the trial was amended to use two doses for the phase 2 portion, and an additional study site was opened. Study accrual is ongoing with a target of 20 additional patients in the phase 2 portion.
Conclusion
This ongoing phase 1b/2 trial of CPX-351 (liposomal cytarabine and daunorubicin) as re-induction treatment for intermediate and high-risk AML has been safe and tolerable with early treatment outcomes that appear to be much better than reported historical controls. Correlative studies by mass cytometry will further establish the mechanistic reasons for the observed responses.
OffLabel Disclosure:
Blachly:Astellas: Consultancy; Epigenetic classification of leukemia: Patents & Royalties: PCT conversion filed; Leukemia Diagnostic Device: Patents & Royalties: Being prosecuted; AstraZeneca: Consultancy; AbbVie: Consultancy. Choe:Actinium Pharmaceuticals: Other: Support for attending meetings and/or travel; MJH Life Sciences: Honoraria; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Receipt of equipment, materials, drugs to institution; Opna: Other: Receipt of equipment, materials, drugs to institution, Research Funding; NIH National Cancer Institute: Research Funding. Vasu:Omeros Inc: Research Funding; Sanofi Inc: Research Funding. Wall:CTI: Speakers Bureau; Abbvie, BMS: Honoraria. Larkin:Gilead: Honoraria; Astellas Pharma: Consultancy; Debiopharm international: Research Funding. Eisfeld:Karyopharm Therapeutics: Other: spouse employment; Astra Zeneca: Honoraria, Other: CEI Advisory Board; OncLive: Honoraria. Borate:Takeda: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; RUNX1 Foundation: Honoraria; Jazz: Other: Research; Pfizer: Other: Research; Genentech: Membership on an entity's Board of Directors or advisory committees; Kura: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Research; Incyte: Other. Mims:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Jonas:AbbVie: Consultancy, Other: travel reimbursement , Research Funding; BMS: Consultancy, Research Funding; Rigel: Consultancy, Other: travel reimbursement ; Genentech/Roche: Research Funding; Treadwell: Research Funding; Loxo: Research Funding; Celgene: Research Funding; F. Hoffmann-La Roche: Research Funding; Aptose: Research Funding; AROG: Research Funding; Incyte: Research Funding; Sigma Tau: Research Funding; GlycoMimetics: Consultancy, Other: protocol steering committee , Research Funding; Jazz: Consultancy, Research Funding; Kymera: Consultancy; Pfizer: Consultancy, Research Funding; Forma: Research Funding; Forty-Seven: Research Funding; Amgen: Research Funding; Servier: Consultancy; Takeda: Consultancy; Pharmacyclics: Research Funding; Hanmi: Research Funding; Immune-Onc: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Gilead: Consultancy, Other: data monitoring committee , Research Funding. Behbehani:Jazz Pharmaceuticals: Research Funding.
Liposomal Daunorubicin and Cytarabine (CPX-351) has been approved by the FDA for treatment of newly-diagnosed therapy-related acute myeloid leukemia or AML with myelodysplasia-related changes in adults and pediatric patients >1 year of age.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal